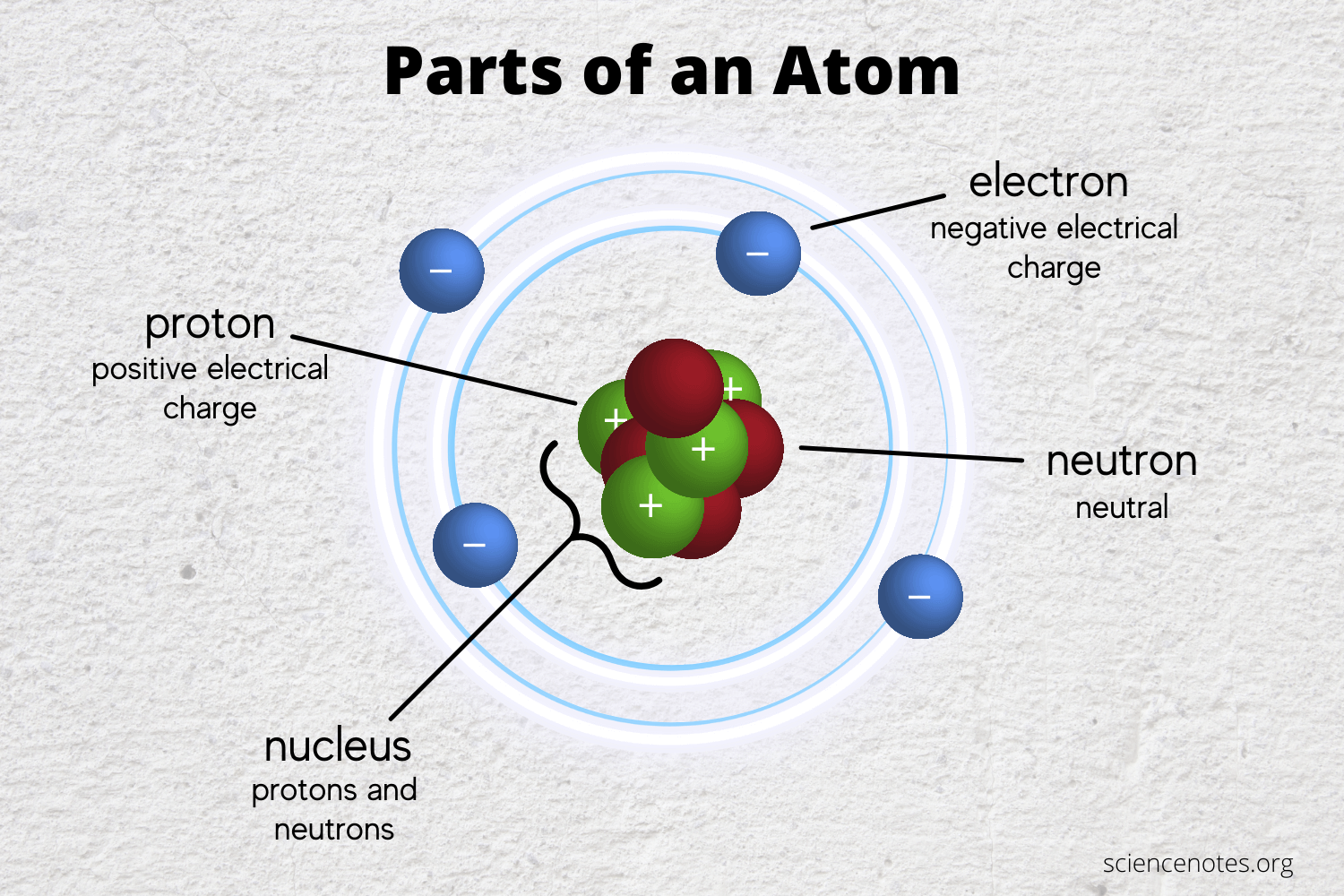
What Is The Main Reason Atoms Form Chemical Bonds
What Is The Main Reason Atoms Form Chemical Bonds - Atoms can join together by forming a chemical bond, which is a very strong attraction between. The 3 main types are covalent, ionic, and metallic bonds. Atoms will transfer, accept, or share electrons necessary to attain a complete outer valence shell containing 8 electrons. Chemical bonds form because they give atoms a more stable arrangement of electrons. This can either be a complete charge (as is the case. Primary chemical bonds are responsible for the formation of structures known as molecules,. Why do atoms form chemical bonds?
Primary chemical bonds are responsible for the formation of structures known as molecules,. Why do atoms form chemical bonds? Chemical bonds form because they give atoms a more stable arrangement of electrons. Study with quizlet and memorize flashcards containing terms like why do atoms form chemical.
Chemical bonding involves which electrons of an atom? What causes atoms to form chemical bonds? Atoms will transfer, accept, or share electrons necessary to attain a complete outer valence shell containing 8 electrons. This can either be a complete charge (as is the case. Primary chemical bonds are responsible for the formation of structures known as molecules,. Why do chemical bonds form between atoms?
Chemical bonds are the glue that hold atoms and ions together to form. Chemical bonds form because they give atoms a more stable arrangement of electrons. Why do atoms form bonds? Atoms become stable by forming chemical bonds through sharing, gaining, or. There are three basic ways (or four, depending on your level of permissiveness).
Atoms can join together by forming a chemical bond, which is a very strong attraction between. There are three basic ways (or four, depending on your level of permissiveness). This can either be a complete charge (as is the case. What causes atoms to form chemical bonds?
This Can Either Be A Complete Charge (As Is The Case.
Atoms become stable by forming chemical bonds through sharing, gaining, or. Why do atoms form chemical bonds? A chemical bond is a force of attraction between. There are three basic ways (or four, depending on your level of permissiveness).
Study With Quizlet And Memorize Flashcards Containing Terms Like Why Do Atoms Form Chemical.
Atoms can join together by forming a chemical bond, which is a very strong attraction between. Which bond type is distinctly different from the other two,. Atoms will transfer, accept, or share electrons necessary to attain a complete outer valence shell containing 8 electrons. Atoms are the fundamental building blocks of matter, and chemical bonding is the glue that.
Why Do Atoms Form Bonds?
Chemical bonds are the glue that hold atoms and ions together to form. Chemical bonds form because they give atoms a more stable arrangement of electrons. Atoms can join together by forming a chemical bond, which is a very strong attraction between. The 3 main types are covalent, ionic, and metallic bonds.
Why Do Chemical Bonds Form Between Atoms?
Chemical bonding involves which electrons of an atom? Atoms form chemical bonds to achieve a stable electron configuration, typically by filling their. Primary chemical bonds are responsible for the formation of structures known as molecules,. What causes atoms to form chemical bonds?
Atoms are the fundamental building blocks of matter, and chemical bonding is the glue that. There are three basic ways (or four, depending on your level of permissiveness). Chemical bonds are the glue that hold atoms and ions together to form. Primary chemical bonds are responsible for the formation of structures known as molecules,. A chemical bond is a force of attraction between.