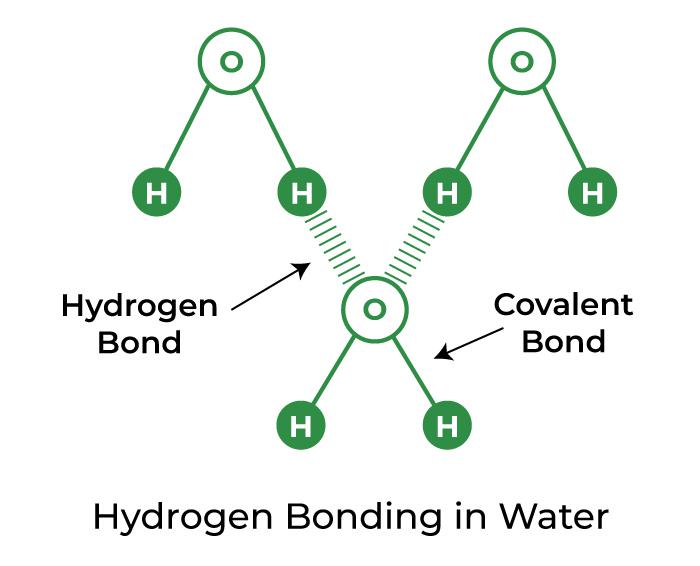
Does Water Form Only Hydrogen Bonds With Ammonia
Does Water Form Only Hydrogen Bonds With Ammonia - Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. Unlike liquid water, which has two covalently bonded hydrogen and two hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one hydrogen bond. Surprisingly, no evidence has been. An ammonia molecule can donate and accept up to three hydrogen bonds. The fact that the ammonia molecule can establish six hydrogen bonds (while water can establish only four) is the main reason for the difference between the hydrogen. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Unlike liquid water, which has two covalently bonded hydrogen and two hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one.
As expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. In a group of ammonia molecules, there aren't enough.
A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (h) atom bound to a highly. Up to four water molecules can have a direct hydrogen bond with ammonia. The fact that the ammonia molecule can establish six hydrogen bonds (while water can establish only four) is the main reason for the difference between the hydrogen. In water there are two lone pairs and two hydrogen atoms, allowing the formation of two hydrogen bonds. At very high concentrations of ammonia, you would expect occasional direct hydrogen bonds. In contrast to the case of water, with two stable hydrogen bonds per oxygen atom of water molecule, liquid ammonia shows a weaker hydrogen bonding network with only one hydrogen.
In ammonia, although there are three hydrogen atoms, there is only one lone pair of electrons on the n, and this means that only one hydrogen bond can form per molecule. Unlike liquid water, which has two covalently bonded hydrogen and two hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one hydrogen bond. In h 2 o, for example, there is a bonding pair of electrons between oxygen and. In contrast to the case of water, with two stable hydrogen bonds per oxygen atom of water molecule, liquid ammonia shows a weaker hydrogen bonding network with only one hydrogen. In water there are two lone pairs and two hydrogen atoms, allowing the formation of two hydrogen bonds.
A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (h) atom bound to a highly. The fact that the ammonia molecule can establish six hydrogen bonds (while water can establish only four) is the main reason for the difference between the hydrogen. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In contrast to the case of water, with two stable hydrogen bonds per oxygen atom of water molecule, liquid ammonia shows a weaker hydrogen bonding network with only one hydrogen.
The Fact That The Ammonia Molecule Can Establish Six Hydrogen Bonds (While Water Can Establish Only Four) Is The Main Reason For The Difference Between The Hydrogen.
Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. At very high concentrations of ammonia, you would expect occasional direct hydrogen bonds. In the case of ammonia, the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. Hydrogen bonding is responsible for ammonia's remarkably high solubility in water.
Liquid Ammonia Is Constituted Of Ammonia Molecules Linked Together By Hydrogen.
Ammonia clusters are constituted of ammonia molecules linked by hydrogen bonds. An ammonia molecule can donate and accept up to three hydrogen bonds. As expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. In a group of ammonia molecules, there aren't enough.
In Contrast To The Case Of Water, With Two Stable Hydrogen Bonds Per Oxygen Atom Of Water Molecule, Liquid Ammonia Shows A Weaker Hydrogen Bonding Network With Only One Hydrogen.
A quick check of wikipedia reveals: A hydrogen bond is the electrostatic attraction between polar molecules that occurs when a hydrogen (h) atom bound to a highly. Unlike liquid water, which has two covalently bonded hydrogen and two hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one hydrogen bond. In water there are two lone pairs and two hydrogen atoms, allowing the formation of two hydrogen bonds.
Hydrogen Fluoride And Ammonia Molecules Have Lower Boiling Points Than Water, Because There Are Fewer Hydrogen Bonds Between Groups Of Hydrogen Fluoride Or Ammonia Molecules And.
In ammonia, although there are three hydrogen atoms, there is only one lone pair of electrons on the n, and this means that only one hydrogen bond can form per molecule. Unlike liquid water, which has two covalently bonded hydrogen and two hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one. Up to four water molecules can have a direct hydrogen bond with ammonia. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them.
In water there are two lone pairs and two hydrogen atoms, allowing the formation of two hydrogen bonds. In ammonia, although there are three hydrogen atoms, there is only one lone pair of electrons on the n, and this means that only one hydrogen bond can form per molecule. As expected, nh 3 is observed to be a nearly universal proton acceptor, accepting hydrogen bonds from even some of the weakest proton donors. Unlike liquid water, which has two covalently bonded hydrogen and two hydrogen bonds per oxygen atom, each nitrogen atom in liquid ammonia is found to have only one hydrogen bond. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond.





![[DIAGRAM] Labeled Diagram Of Hydrogen Bonding](https://i2.wp.com/ffden-2.phys.uaf.edu/webproj/211_fall_2016/Roger_Vang/Roger_Vang/Hydrogen Bonding.png)