Do Metals Form Cations Or Anions
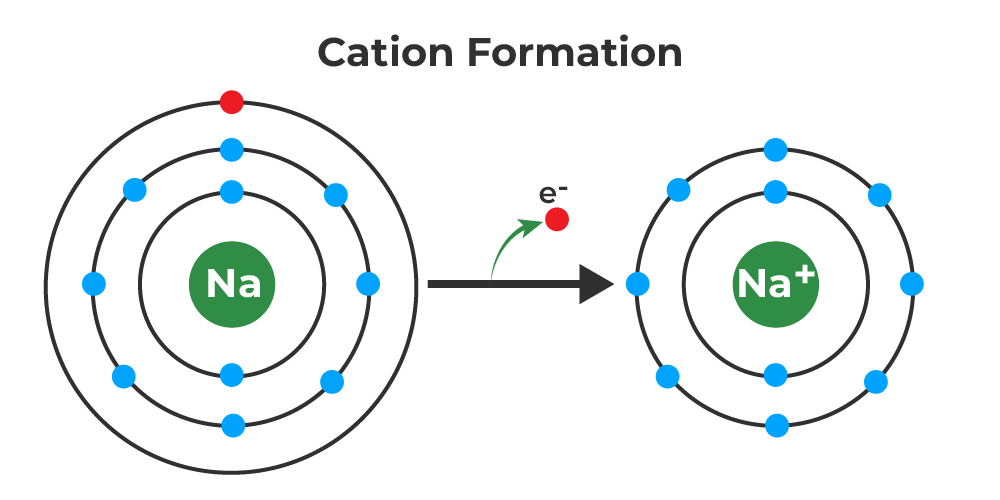
Do Metals Form Cations Or Anions - With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. For example, nacl is a binary ionic compound. What is the difference between. Metals tend to form cations, while nonmetals tend to form anions. For example, nacl is a binary ionic compound. Thus metals are electropositive elements with relatively. The alkali metals tend to form +1 cations.
Binary ionic compounds are composed of just two elements: Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. For example, nacl is a binary ionic compound.
This difference in charge is what. The following periodic table shows some of the common ions formed by common elements. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. First, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. This is actually one of the chemical properties of metals and. A metal (which forms the cations) and a nonmetal (which forms the anions).
The alkali metals tend to form +1 cations. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. Binary ionic compounds are composed of just two elements: Thus metals are electropositive elements with relatively. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.
What is the difference between. The following periodic table shows some of the common ions formed by common elements. Understand the major difference between cations and anions. This difference in charge is what.
This Is Actually One Of The Chemical Properties Of Metals And.
Metals tend to form cations, while nonmetals tend to form anions. A metal (which forms the cations) and a nonmetal (which forms the anions). First, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal.
For Example, Nacl Is A Binary Ionic Compound.
Understand the major difference between cations and anions. This is actually one of the chemical properties of metals and. The alkali metals tend to form +1 cations. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high.
A Metal (Which Forms The Cations) And A Nonmetal (Which Forms The Anions).
For example, nacl is a binary ionic compound. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Thus metals are electropositive elements with relatively. Binary ionic compounds are composed of just two elements:
Binary Ionic Compounds Are Composed Of Just Two Elements:
This difference in charge is what. Metals tend to form cations and nonmetals tend to form anions. The following periodic table shows some of the common ions formed by common elements. What is the difference between.
This is actually one of the chemical properties of metals and. A metal (which forms the cations) and a nonmetal (which forms the anions). This is actually one of the chemical properties of metals and. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the cation) and the high. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal.