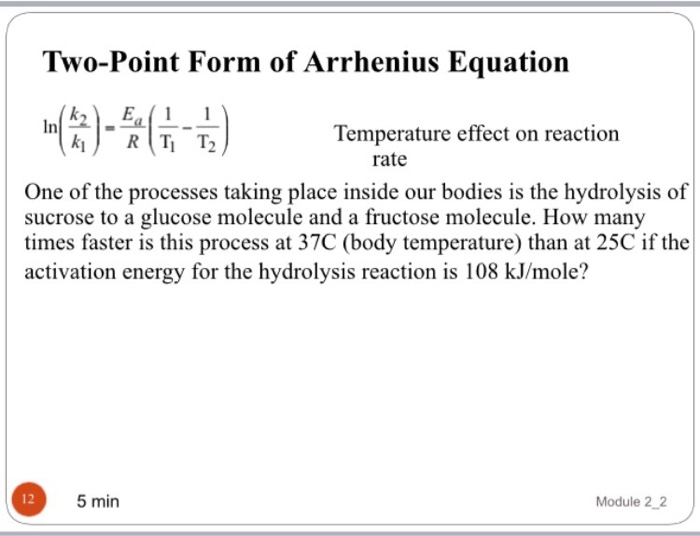
Arrhenius Equation Two Point Form
Arrhenius Equation Two Point Form - This video shows how to calculate values using the arrhenius equation when there is not a frequency factor. To get started, identify the values provided in the problem, which include t 2 = 525 k, t 1 = 701 k, k 1 = 2.57 l ⋅ mol − 1 ⋅ s − 1, and activation energy e a =. Use two (2) point slope form calculator to find the equation of straight line passing through two points. The arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. Calculate the value for the arrhenius energy of activation, ea, for reaction 1, below. Enter both points in input boxes to get the equation of line. Here’s the best way to solve it.
To get started, identify the values provided in the problem, which include t 2 = 525 k, t 1 = 701 k, k 1 = 2.57 l ⋅ mol − 1 ⋅ s − 1, and activation energy e a =. Many reactions that take place at. Here’s the best way to solve it. The arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast.
The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. To get started, identify the values provided in the problem, which include t 2 = 525 k, t 1 = 701 k, k 1 = 2.57 l ⋅ mol − 1 ⋅ s − 1, and activation energy e a =. Many reactions that take place at. Here’s the best way to solve it. Assuming first order arrhenius equation | use second order arrhenius equation instead This video shows how to calculate values using the arrhenius equation when there is not a frequency factor.
The following data shows the rate constant of a reaction measured
The arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. This video shows how to calculate values using the arrhenius equation when there is not a frequency factor. The energy of activation for this reaction is quite high. Enter both points in input boxes to get the equation of line. Many reactions that take place at.
Enter both points in input boxes to get the equation of line. Many reactions that take place at. Here’s the best way to solve it. Calculate the value for the arrhenius energy of activation, ea, for reaction 1, below.
To Get Started, Identify The Values Provided In The Problem, Which Include T 2 = 525 K, T 1 = 701 K, K 1 = 2.57 L ⋅ Mol − 1 ⋅ S − 1, And Activation Energy E A =.
The arrhenius equation for the activation energy of a chemical reaction, especially its two point form, is an intimidating looking beast. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants. Assuming first order arrhenius equation | use second order arrhenius equation instead Here’s the best way to solve it.
Use Two (2) Point Slope Form Calculator To Find The Equation Of Straight Line Passing Through Two Points.
Many reactions that take place at. Calculate the value for the arrhenius energy of activation, ea, for reaction 1, below. We have two rate constants and two temperatures. Enter both points in input boxes to get the equation of line.
The Energy Of Activation For This Reaction Is Quite High.
This video shows how to calculate values using the arrhenius equation when there is not a frequency factor.
Calculate the value for the arrhenius energy of activation, ea, for reaction 1, below. The energy of activation for this reaction is quite high. This video shows how to calculate values using the arrhenius equation when there is not a frequency factor. Use two (2) point slope form calculator to find the equation of straight line passing through two points. The arrhenius activation energy for two temperature calculator uses the arrhenius equation to compute activation energy based on two temperatures and two reaction rate constants.